About MITRO
July 3, 2025, the official website of the CDE showed that, Hexin (Suzhou) Pharmaceutical Technology Co., Ltd. (hereinafter referred to as "Hexin Pharmaceutical") independently developed 1 a class of radiotherapeutic drugs 177Lu-HX02 injection has been officially approved for clinical use, for the treatment of integrin αvβ3 and / or CD13 receptor-positive tumors Following 2022 the year's first 68 Ga diagnostic product was approved for clinical use, Hexin Pharmaceutical has again made a breakthrough in the treatment products of the same target point, reflecting the company's profound understanding of the integrated diagnosis and treatment of radioisotope-conjugated drugs (RDC).
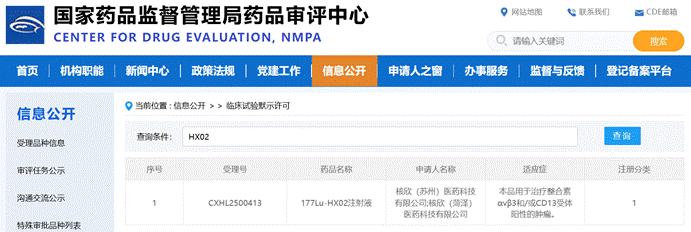
Mitro Biotech Co., Ltd. (hereinafter referred to as "Mitro") is very honored to have, as a partner of Hexin Pharmaceutical, deeply participated in 177Lu-HX02 injection IND application's key links, relying on its deep accumulation and professional technical platform in the field of radiopharmaceutical research and development, Mitro undertook and completed the pharmaceutical research and non-clinical research work for 177Lu-HX02 injection , contributing to the smooth progress of the project.
We are well aware of the hardships and difficulties of innovative drug research and development, and sincerely congratulate the Hexin Pharmaceutical team on this important milestone! Mitro is always committed to becoming a trustworthy partner for radiopharmaceutical R&D companies, providing CRDMO services covering the entire chain of radiopharmaceutical R&D.
We look forward to 177Lu-HX02 the smooth progress of the subsequent clinical trials of the injection, and hope that it will benefit patients as soon as possible! Mitro will also continue to improve its technology and optimize its services to help more partners accelerate the R&D and listing process of innovative radiopharmaceuticals!
Related News


