About MITRO
On September 22, 2025, the Class 1 new drug 225Ac-LNC1011 Injection, developed by Yantai Lannacheng Biotech Co., Ltd. ("Lannacheng"), obtained implicit approval for clinical trials from China's Center for Drug Evaluation (CDE). This milestone follows the prior receipt of a Clinical Trial Approval from the U.S. Food and Drug Administration (FDA), marking a significant achievement of dual regulatory approvals in both major markets. Throughout this application process, MITRO Biotech Co., Ltd. ("MITRO") was entrusted to provide comprehensive pharmaceutical research and non-clinical support services, including toxicology studies, for the project.
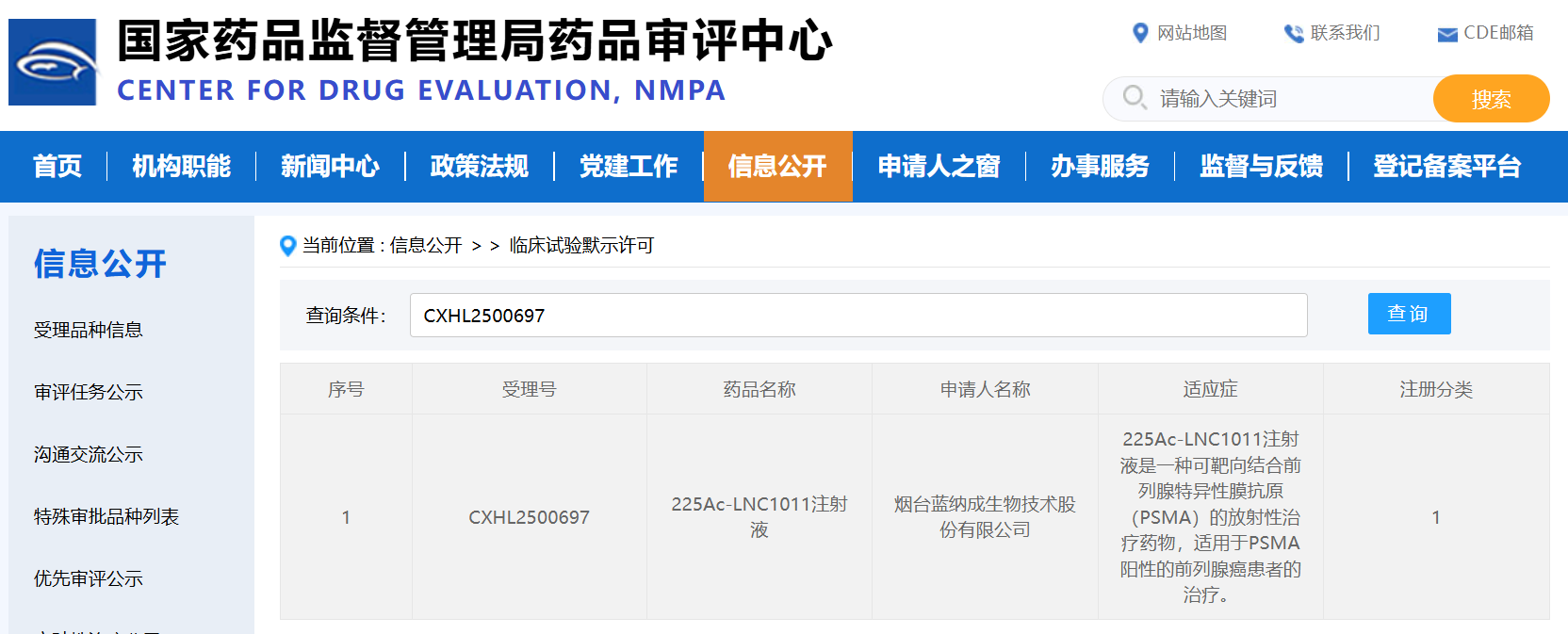
225Ac-LNC1011 Injection is a targeted alpha-particle therapy radiopharmaceutical directed against Prostate-Specific Membrane Antigen (PSMA). It is intended for the treatment of patients with advanced PSMA-positive prostate cancer. Compared to traditional beta-emitters, alpha-particles are characterized by a short path length (<100 μm), high linear energy transfer (80-100 keV/μm), and the emission of multiple alpha decays along the decay chain. These properties enable potent and localized tumor cell killing while potentially minimizing damage to surrounding healthy tissues.
PSMA is a type II transmembrane glycoprotein highly and specifically expressed on the surface of prostate cancer cells and metastatic lesions. In completed preclinical and clinical studies, 225Ac-LNC1011 Injection has demonstrated favorable pharmacokinetics and significant antitumor efficacy, showcasing its potential as a promising, effective, and precise therapeutic option for patients with metastatic castration-resistant prostate cancer (mCRPC).
About Lannacheng:
Yantai Lannacheng Biotech Co., Ltd. was established in early 2021, with its headquarters in Yantai, China, and a subsidiary in Singapore. Founded by Dongcheng Pharmaceutical Group, a leading enterprise with significant influence in China’s nuclear medicine sector, Lannacheng is dedicated to the research, development, and registration of first-in-class innovative radiopharmaceuticals integrating diagnosis and therapy. The company is developing various theranostic radiopharmaceutical pipelines with strong translational potential, serving as a core part of Dongcheng’s nuclear medicine ecosystem.
About MITRO:
Established in 2012, MITRO Biotech Co., Ltd. focuses on providing professional and efficient CRO and CDMO services of radiolabeling and molecular imaging to the global pharmaceutical and biopharmaceutical industries. With Radiolabeling and Molecular Imaging technology, MITRO provides outsourcing technical services such as drug screening, biodistribution, PK study and PD evaluation for pharmaceutical enterprises and research institutes at home and abroad. It also provides one-stop solutions for new drug R&D, including labeling method development, pharmacological studies, non-clinical studies, clinical studies, and registration application. Our business focuses on studies such as oncological, nervous system diseases, autoimmune diseases, cardiovascular and cerebrovascular diseases, as well as innovative therapies such as immunity, gene therapy, and cell therapy, so as to explore disease mechanisms, developing diagnostic and therapeutic drugs, and evaluate therapeutic effects.
Previous article
Related News


